Why measure insulin and glucagon for studying muscle maintenance?
While insulin resistance still may lead to multiple diseases such as diabetes and cardiovascular disease, insulin sensitivity has been associated with greater muscle mass.
Discover glucagon’s vital role in metabolic balance, its interaction with insulin, and its implications in metabolic diseases. Learn about the measuring challenges of this crucial peptide and why Mercodia’s Glucagon ELISA is a reliable method.
This article was updated in March 2025.
Glucagon is a linear peptide containing 29 amino acids, secreted by the α-cells in the pancreas, and it is essential for metabolic homeostasis regulation. This molecule, isolated by Charles Kimball and John Murlin in 1922, was purified and sequenced (in 1953 and 1957 respectively) by Eli Lilly and measured for the first time in 1959 at Roger Unger’s lab [ref. 1].
Glucagon is a molecule that circulates in the low picomolar range [ref. 2]. It has been well established that it responds differently depending on the meal composition, with carbohydrate-rich meals inhibiting glucagon secretion, and increasing with the protein content of the meal stimulating it [ref. 3].
It is important to remember that glucagon has diverse effects on multiple organs, such as the pancreas, liver, kidney, heart, and brain. Although its actions have not been well established in all organs, the pharmacological perspective of glucagon’s role remains relevant [ref. 1].
Glucagon’s role in intra-islet paracrine regulation is essential, thus, glucagon release is important for insulin secretion and vice versa [ref. 1, 4]. Stimulation of β-cell via glucagon binding to the glucagon receptor (GCGR) as well as the glucagon-like peptide 1 (GLP-1) receptors is essential for amino acid-induced insulin secretion [ref. 1].
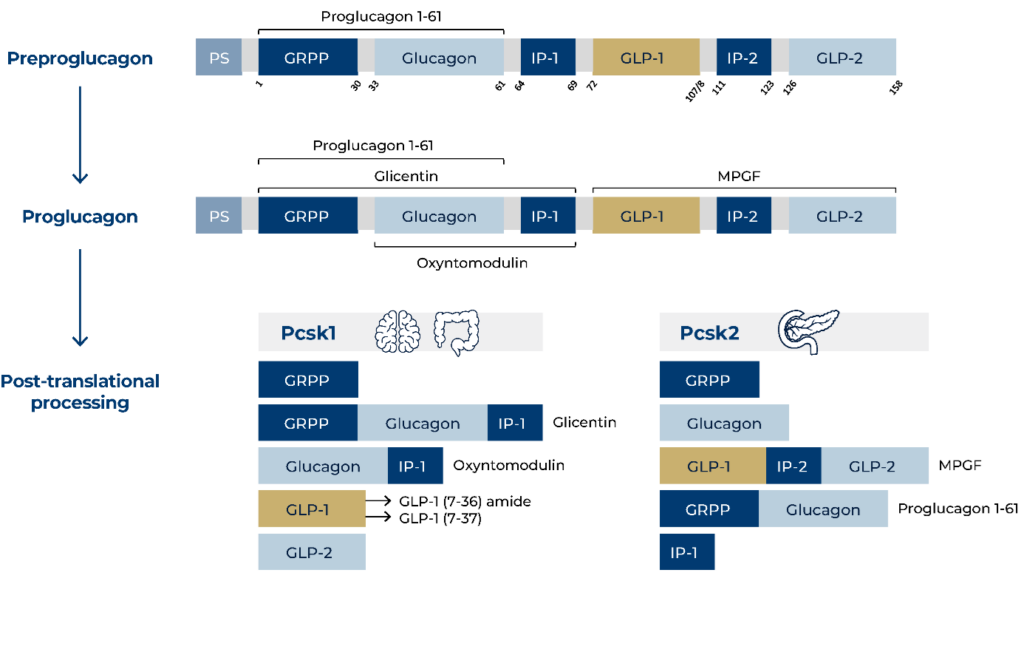
PS: Signal peptide; GRPP: glicentin-related polypeptide; IP-1: intervening peptide-1; GLP-1: glucagon-like peptide-1; IP-2: intervening peptide-2; GLP-2: glucagon-like peptide-2; MPGF: major proglucagon fragment; PCSK1: prohormone convertase 1/3; PCSK2: prohormone convertase 2.
In healthy individuals, high blood glucose stimulates β-cell insulin release and inhibits glucagon secretion; conversely, low blood glucose inhibits β-cell insulin and stimulates glucagon secretion [ref. 4]. It has been shown that glucagon’s concentration during fasting (around 10 pmol/L, which corresponds to a 2–3 fold higher concentration in the portal vein), is essential for hepatic glucose production during the fasting and inter digestive phases [ref. 3]. In patients, hyperglucagonaemia has been reported in various conditions including type 2 diabetes, type 1 diabetes, obesity, fatty liver diseases, and kidney diseases, where increased glucagon may be explained by increased secretion, decreased degradation/extraction/clearance, and inaccurate measurements due to assay cross-reactivity with other glucagon-like molecules.
Moreover, the settings where hyperglucagonaemia is observed must be considered (fasting conditions, or postprandially after an oral glucose tolerance test or mixed meal) [ref. 1].

The biosynthesis of glucagon has an impact on its complexity and measurement. It is important to keep in mind that there are at least five glucagon-like molecules (and perhaps more) circulating in the body, creating great demands on the selectivity of a glucagon assay [ref. 1]. Thereby, the sandwich ELISA technology, when strictly based on N- and C-terminal-binding antibodies, can solve these issues making it the ideal solution to the specificity problem of the glucagon measurement [ref. 1, 2].
To evaluate the performance of a glucagon assay it is essential to have several parameters in mind: precision, sensitivity, specificity, and accuracy [ref. 2].
Mercodia’s Glucagon ELISA (10-1271-01) has been shown by multiple researchers and clinicians worldwide to have superior sensitivity, high specificity, minimal matrix interference, plus a low sample volume required. Completing all these requirements has made the Mercodia assay a tool that fulfils the acceptability criteria of multiple studies (e.g., Kahn SE, et al., 2021 [ref. 5]).
Download the Assay Guide – How to choose your Glucagon ELISA

We gladly support you by keeping you updated on our latest products and the developments around our services.