Cusabio recombinant antibodies
Over 600 recombinant antibodies help your breakthroughs in COVID-19, cancer & autoimmune diseases
ADC drug development process includes target selection, antibody discovery & optimization, quality control, etc. Cusabio provides tailored products, services, and solutions to meet researchers’ needs at every stage.
Antibody-Drug Conjugates (ADCs) are innovative pharmaceuticals that combine small molecule drugs with antibodies to target cancer cells precisely. The development of ADCs involves several critical stages: target selection, antibody discovery, quality control, and clinical research. Cusabio, with its extensive experience in protein, antibody, and ELISA kit development, offers tailored solutions for each stage of ADC development.
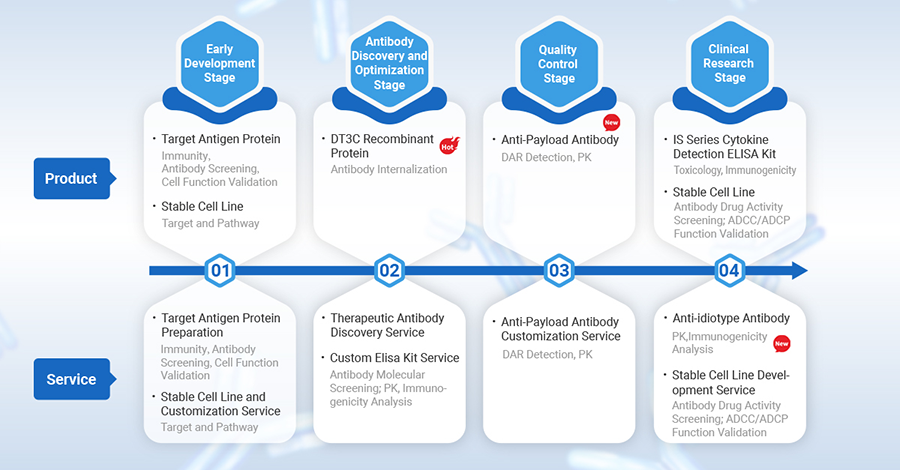
In the early stages, the focus is on generating antibodies that bind specifically to tumor cell antigens. This requires the preparation of highly pure and active target antigen proteins, which are critical for screening and validating antibody specificity, assessing in vitro cytotoxicity, and conducting cell function validations. Stable cell line construction is also essential for target and pathway research. Cusabio offers a range of target antigen protein products and customized services to support these needs.
The goal of this stage is to develop high-affinity, highly specific monoclonal antibodies targeting specific antigens. This is the basis on which ADC drugs are designed to achieve precise targeting and efficient eradication of cancer cells. Cusabio offers DT3C recombinant proteins to assess antibody internalization efficiency, enhancing the selection process. Their Therapeutic Antibody Discovery Service provides a comprehensive solution from target identification to therapeutic antibody candidates. With extensive experience in protein and antibody research, they have screened over 100 therapeutic antibody candidates.
Quality control ensures that the ADC’s payload effectively targets and kills cancer cells. Anti-payload antibodies are used for immunological analysis of the payload in ADC drugs to assess stability, safety, and effectiveness. Cusabio offers specialized anti-payload antibodies (such as Anti DXD mAb, Anti MMAE, Anti Eribulin) for preclinical and clinical stage research, including plasma/serum pharmacokinetic analysis, drug binding affinity determination, DAR value analysis, and ADC drug efficacy evaluation.
In the clinical research stage, Cusabio provides Cytokine Detection ELISA kits for toxicology studies and cellular immune assessments. They also offer stable cell line products and custom services for antibody drug activity screening and ADCC/ADCP functional verification. Additionally, they provide custom anti-idiotype antibody services for pharmacokinetic and immunogenicity analysis. Their IS series of Cytokine detection assay kits is designed for industrial clients, including pharmaceutical companies, for applications such as CRS detection, residual process material testing, and pharmacokinetic experimental cell function evaluation.
We gladly support you by keeping you updated on our latest products and the developments around our services.