GeneTex’s recombinant antibodies for cancer research
GeneTex employs a state-of-the-art production platform and enhanced validation strategies to create reliable and inexhaustible IHC-validated recombinant monoclonal antibodies for cancer research.
GeneTex is using its recombinant monoclonal antibody production platform and enhanced validation strategies to create reliable reagents for G protein-coupled receptor (GPCR) research.
This news was updated in March 2025.
G protein-coupled receptors (GPCRs) represent the largest superfamily of cell surface transmembrane receptors in the human genome (approximately 821 genes). They are characterized by a seven-transmembrane structure linked by both intra- and extracellular loops, with extracellular N-terminus and intracellular C-terminus domains. Signaling is triggered by the binding of various types of ligands, including hormones, neurotransmitters, metabolites, ions, photons, and mechanical forces. Both G protein-dependent and -independent mechanisms are involved in initiating signal transduction with subsequent widespread physiological and pathophysiological effects. Presently, perhaps one-third of prescribed drugs modulate GPCR activity in some way, though it is thought that 60-85% of potentially therapeutic GPCRs have no drugs (1). In fact, the U.S. NIH “Illuminating the Druggable Genome Program” identified GPCRs, ion channels, and kinases as three druggable and yet understudied protein families (2). Therefore, developing high-quality GPCR antibodies is crucial for academic, clinical, and pharmaceutical research. However, producing antibodies against GPCRs has been extremely challenging because of high conformational variability, difficulties with GPCR immunogen preparation, and structural constraints frequently associated with membrane proteins (3).
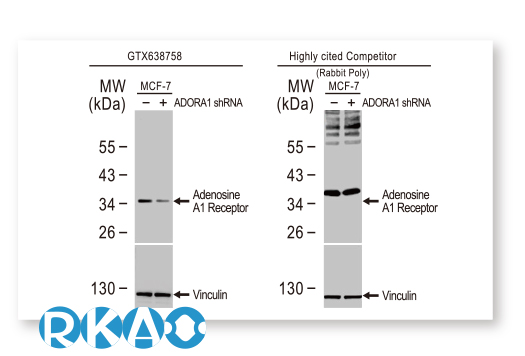
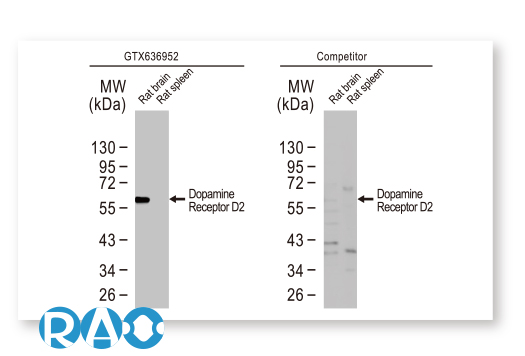
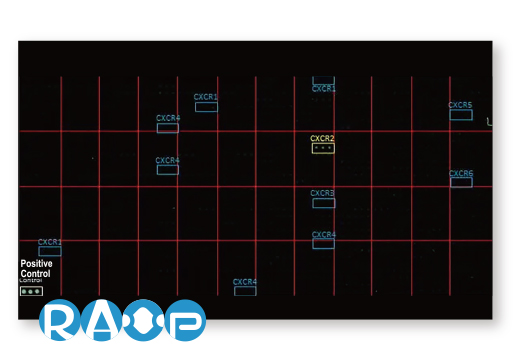
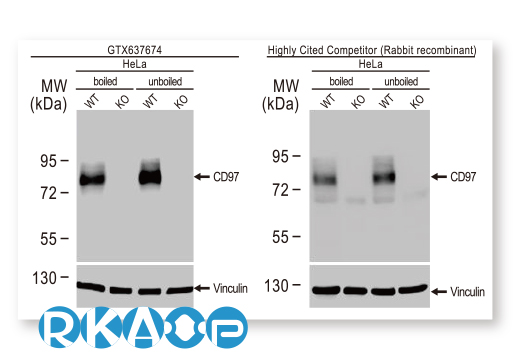
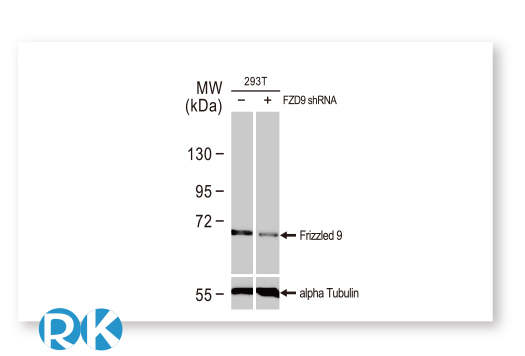
GeneTex is leveraging the advantages of its recombinant monoclonal antibody production system and enhanced “Five Pillar” validation strategies to produce a catalog of best-in-class GPCR antibodies to facilitate research. Meticulous immunogen sequence selection combined with knockout/knockdown testing, comparable antibodies, differential tissue expression, GPCR overexpression, and application-specific testing in immunohistochemistry, immunocytochemistry, and immunoblot are being performed to thoroughly validate these reagents. GeneTex is also utilizing the innovative VirDTM-GPCR array technology (CDI Labs, Baltimore, MD) as a specialized tool to establish antibody specificity for a particular GPCR.
GeneTex’s goal is to target the ~360 human GPCRs that are not olfactory, visual, or taste receptors for recombinant monoclonal antibody production. This number includes perhaps 140 GPCRs that are considered to be orphan receptors. Presently, around 21 well-characterized reagents have already been added to the catalog, with a goal of at least 100 within this year. Over the longer term, GeneTex hopes to expand its efforts beyond GPCRs to other challenging membrane proteins.

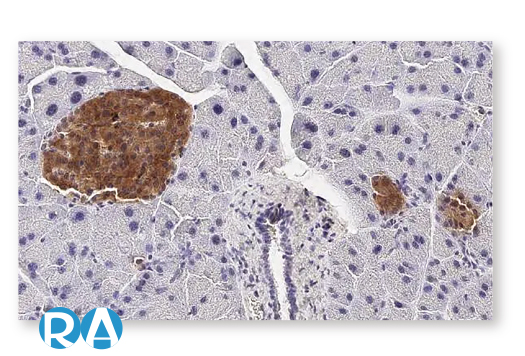
| Glucagon Like Peptide 1 Receptor (GLP1R) |
| Vasoactive Intestinal Peptide Receptor 2 (VPAC2) |
| Adhesion G Protein-Coupled Receptor E5 (CD97) |
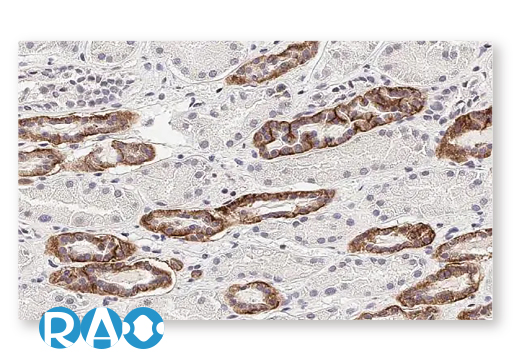
| Calcium Sensing Receptor (CASR) |
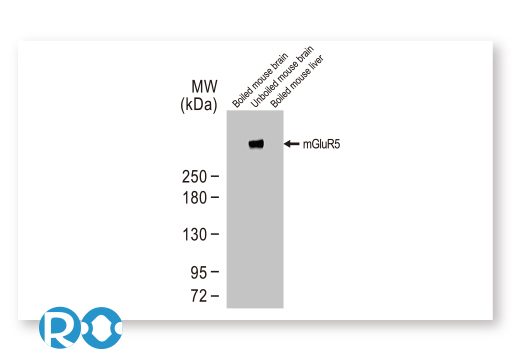
| Glutamate Metabotropic Receptor 5 (mGluR5) |
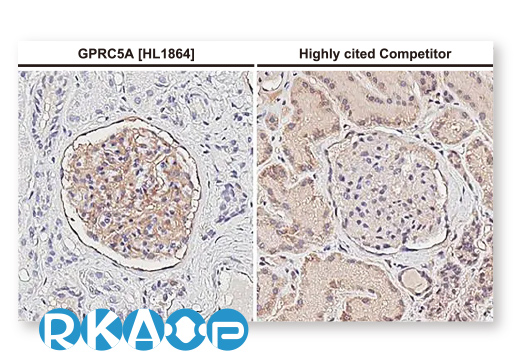
| G Protein-Coupled Receptor Class C Group 5 Member A (GPRC5A) |
References:
We gladly support you by keeping you updated on our latest products and the developments around our services.