Calprotectin – The superior Inflammatory Biomarker
Calprotectin has many other important uses. Download the free white paper and find out what makes it an excellent biomarker for complement-based indications.
Explore the complement pathway in a whole new way with Svar’s quantitative Factor P assay and the first commercially available functional Factor P assay.
Factor P, also referred to as properdin, has an important role in the amplification loop of the complement system. Upon binding to C3bBb, it acts as a stabilizer, extending the half-life of C3bBb significantly.
In addition, it inhibits the factor H-mediated cleavage of C3b by factor I, thereby increasing C3b formation and positively regulating the complement cascade response.
As an up-regulator of the complement system, properdin is of interest to pharmaceutical companies looking for therapeutic targets, as well as to academic groups studying the amplification loop.
Svar Life Science is happy to announce the launch of two new ELISA-based Factor P assays:
These assays allow you to accurately measure the levels of properdin and determine its functionality. Moreover, since the assays use the same capture antibody, you can directly relate the properdin concentration and function in your samples.
In addition, the assays can easily be adapted to more in-depth studies, granting a closer look at the amplification loop.
In the quantitative assay, the wells are coated with a capture antibody that binds to Factor P (FP) in the sample. An enzyme-conjugated detection antibody and a corresponding substrate are used to generate a signal corresponding to the properdin concentration in the sample.
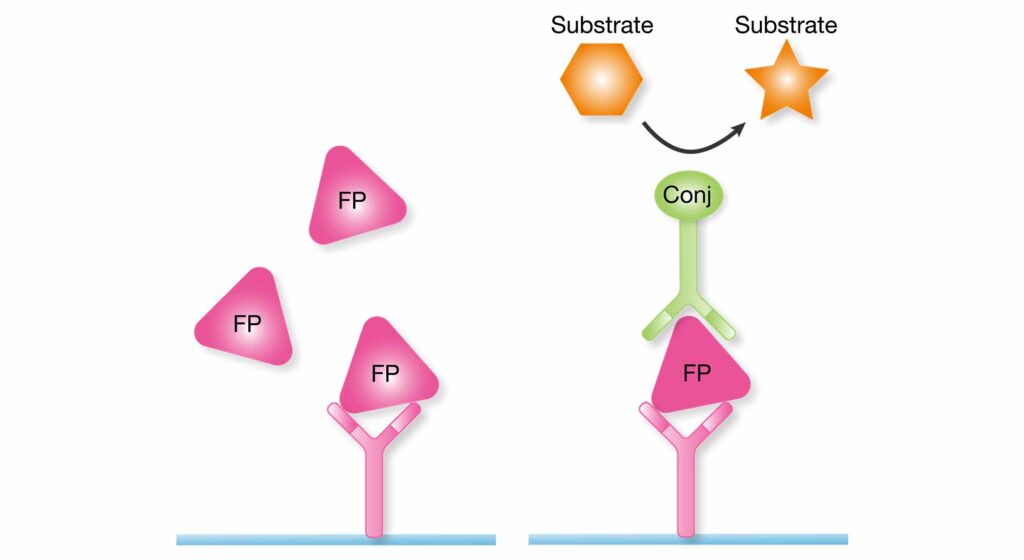
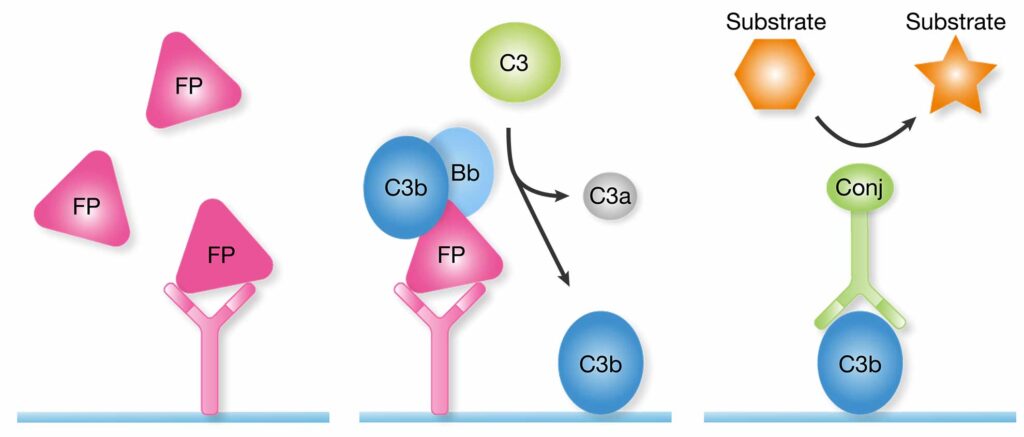
Factor P (FP) is bound by the same capture antibody in the functional assay as in the quantitative assay. A reference serum is then added. If the initial sample contains functional properdin, C3 from the reference will be cleaved to C3b. An enzyme-conjugated detection antibody is used to detect C3b in the well.
We gladly support you by keeping you updated on our latest products and the developments around our services.