SARS-CoV-2 and calprotectin
COVID-19, the disease caused by the SARS-CoV-2 virus, has stretched hospital and ICU resources to their limits in almost every country, and is so far responsible for 4.5 million documented deaths.
Neutralizing antibodies are antibodies with the highest level of protectability against reinfection or serious course of the disease.
Beckman Coulter offers SARS–CoV–2 ProtectAbility EIA – a simple quantitative ELISA assay that may serve as substitution for conventional Virus neutralization test.
Typical immunoassays use as antigenic determinants various viral proteins, particularly those which are engaged in host cell penetration – S protein or its parts containing receptor binding domain (RBD) of SARS-CoV-2. Nevertheless, such systems detect also antibodies without any neutralizing effect – so called binding antibodies. It is true even if amino acid sequence corresponding to RBD itself is used.
SARS-CoV-2 ProtectAbility EIA employs, besides a protein containing RBD, also defined mouse monoclonal antibody (2D11) against RBD. Unique principle of the assay enables selective determination of only those antibodies, which are really neutralizing.
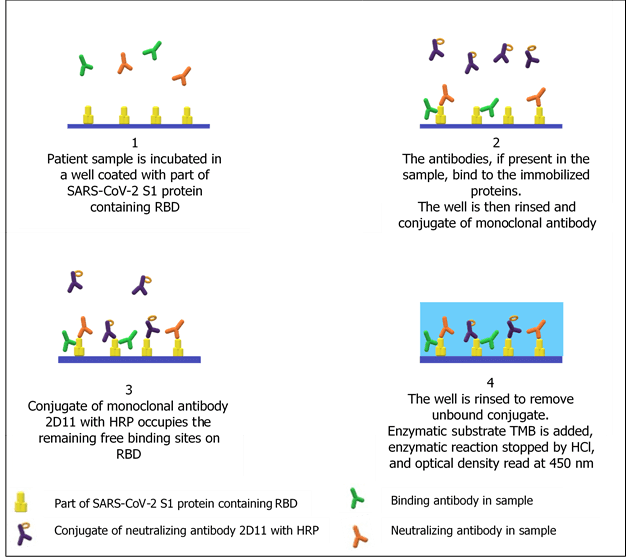
The principle of the assay makes it particularly suitable for different mutations of SARS–CoV–2. The ability of used monoclonal antibody 2D11 to neutralize various mutations of SARS–CoV–2 was confirmed also for SARS-CoV-2-B.1.351 (β-variant), SARS-CoV-2-P.1 (γ-variant) and SARS-CoV-2-B1.617.2 (δ-variant. The testing of its suitability for SARS-CoV-2-B.1.1.529 (o-variant; omicron variant) is currently in process.
The assay is calibrated against international standard WHO 1st IS 20/136, and concentration is reported in IU/mL. 1 IU numerically corresponds to 1 BAU (binding antibody unit), determined by above mentioned assays for the determination of binding antibodies instead of neutralizing.
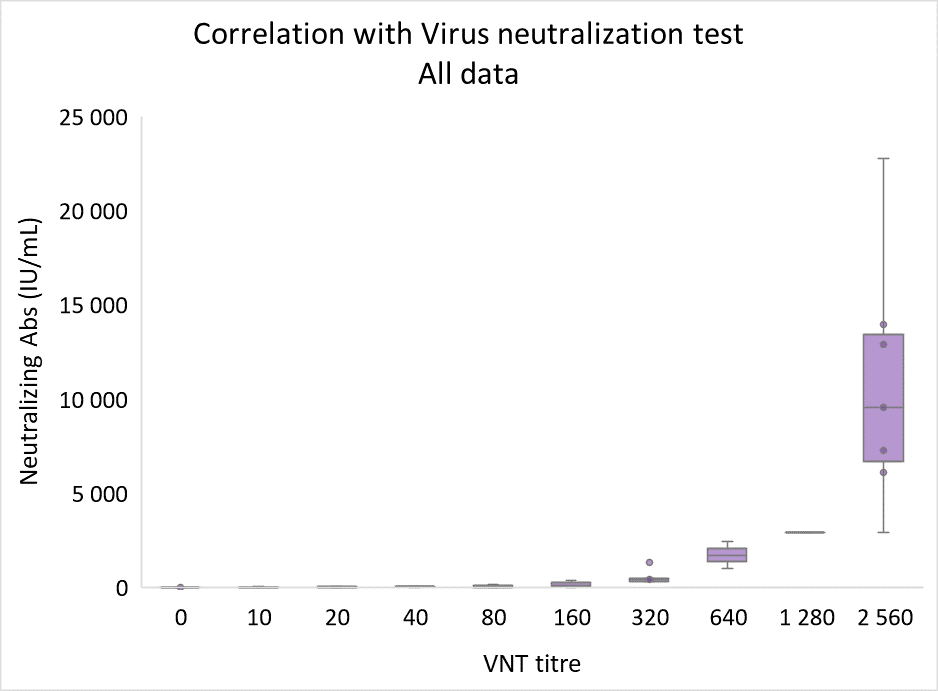
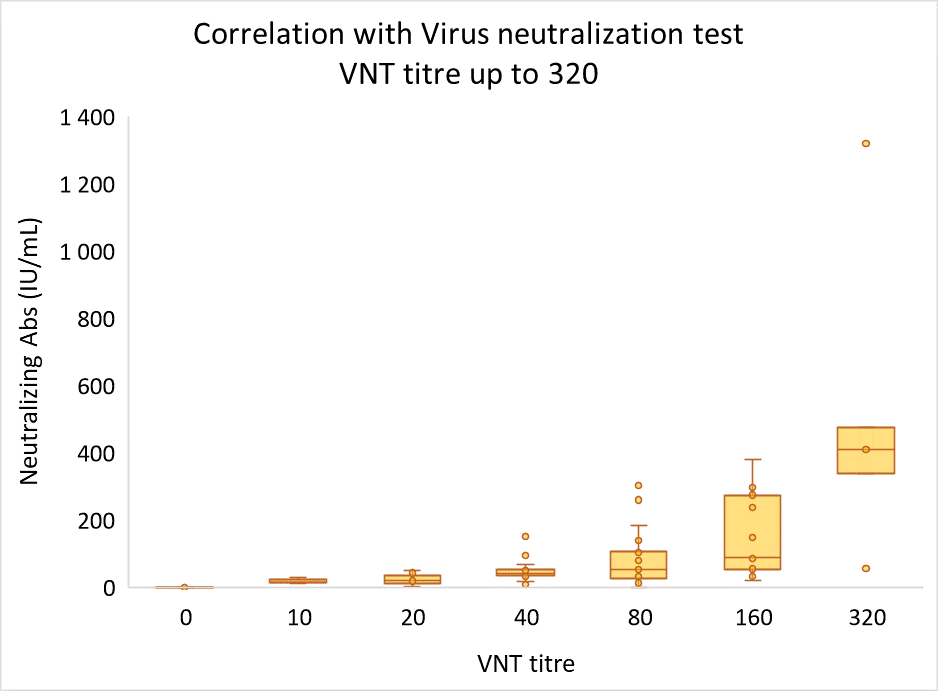
The values corelates well with VNT not only as far as positivity-negativity status is concerned, but also when concentrations are correlated with VNT titers. SARS–CoV–2 ProtectAbility EIA assay provides rapid and relatively easy-to-perform alternative to virus neutralizing test, which may be used as an aid in diagnosis of previous SARS-CoV-2 infection and for the assessment of immune response efficacy.
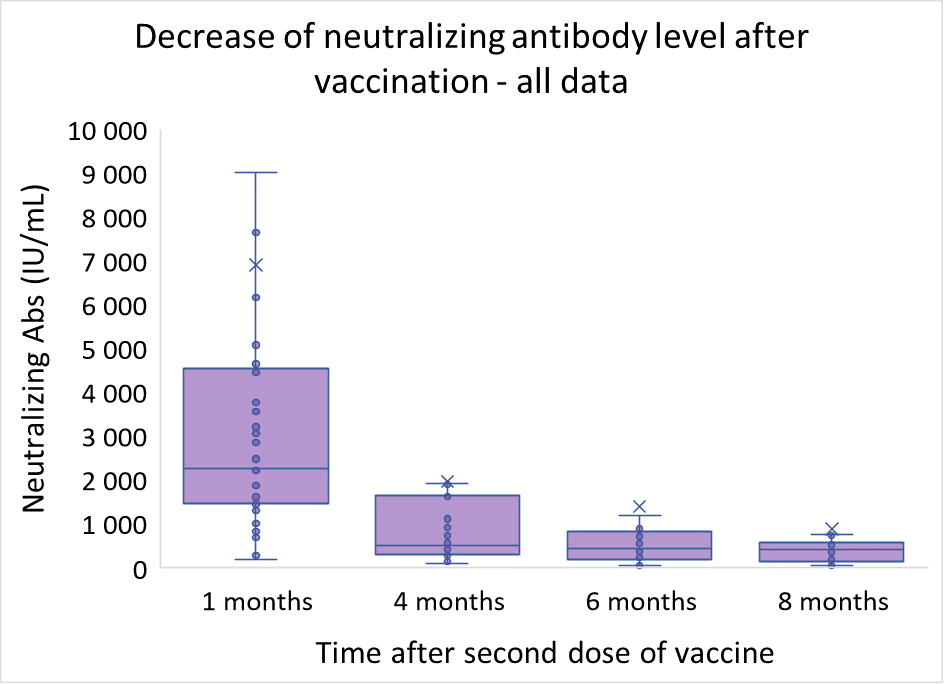
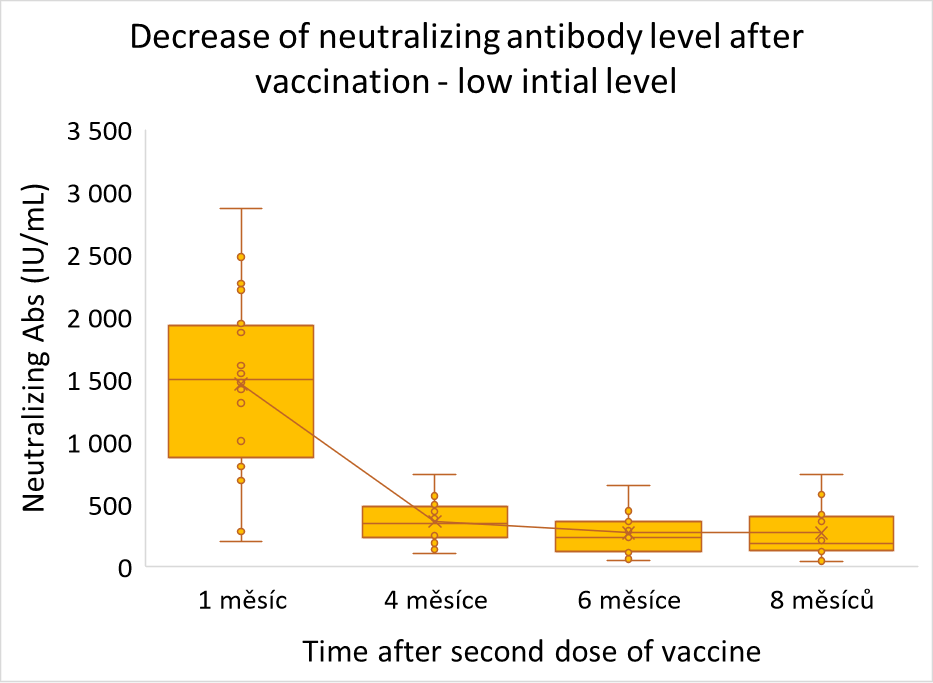
Neutralizing antibodies change after vaccination shows an interesting pattern. The general trend is sustainable decrease in the course of first 8 months after vaccination. Nevertheless, focus on individuals with low initial antibody value (<3 000 IU/mL) shows certain stabilization of antibody values six months after vaccination, though the level at which the concentration stabilizes is very individual.
| REF # | C77994 |
| Principle | Two step EIA |
| Target | SARS–CoV–2 neutralization antibodies, all Ab classes |
| Sample type | Serum, EDTA plasma |
| Sample volume | 25 mL |
| Incubation | 2 x 1 hr/shaking + 10 min |
| Traceability | WHO International Standard 1st IS 20/136 |
| Calibration | 5 point calibration, range 0, 30 – 400 IU/mL Based on defined monoclonal antibody 2D11 |
| Controls | 2 levels (low, high) |
| LoD | 16.8 IU/mL |
| Cut-off | 18.0 IU/mL |
| Clinical specificity | 100.0% |
| Clinical sensitivity | 92.0% |
For more information please contact our technical support.
We gladly support you by keeping you updated on our latest products and the developments around our services.