LeukoStrat® CDx FLT3 Mutation Assay
The only CE-IVD marked assay for selection of acute myeloid leukemia (AML) patients eligible for treatment with midostaurin or gilteritinib fumarate.
NGS Analysis of Hematology-Oncology Clonality Testing
This news was updated in April 2024
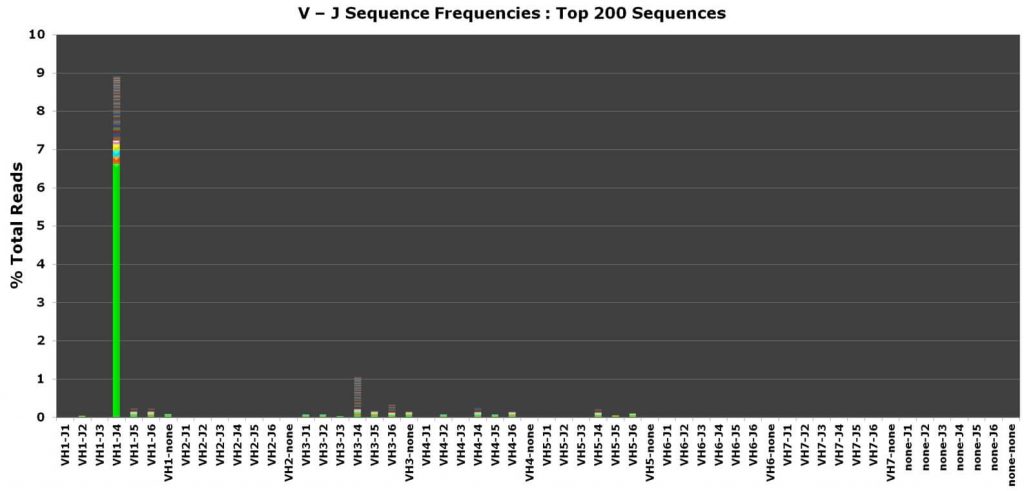
Invivoscribe’s NGS LymphoTrack® Dx Assays are used to detect clonal gene rearrangements, somatic hypermutations and for the study of measurable residual disease (MRD). The full clonality suite of LymphoTrack® Dx Assays are CE-marked and developed for use with the leading NGS platforms, including optimized multiplex PCR master mixes with primers incorporating platform-specific adapters and specimen tracking sequencing identification tags for a one-step PCR workflow. A comprehensive bioinformatics software package is provided free of charge with purchase; enabling you to identify the DNA sequence, clonal prevalence, V-J family identity for each gene rearrangement, and with the IGHV Leader or IGH FR1 assay, the extent of IGHV somatic hypermutation (SHM).
We gladly support you by keeping you updated on our latest products and the developments around our services.